🔬 Retinal Research — Photoreceptors
When a Sensory Organelle Loses Its Supply Line
What are photoreceptors?
Photoreceptors are specialized sensory neurons in the retina responsible for capturing light and initiating visual signaling. Their defining structure, the outer segment, contains tightly stacked membranous discs enriched in phototransduction proteins. This outer segment is a highly modified primary cilium—an organelle that lacks intrinsic protein synthesis machinery and therefore relies entirely on a continuous supply of proteins and lipids from the inner segment and cell body. Disruption in this transport process underlies many inherited retinal degenerative disorders.
The core problem:
If the cilium's import/transport system falters, essential proteins (like rhodopsin, your primary light receptor) don't reach the outer segment. Structure collapses, and vision fades.
What I discovered:
INPP5E & lipids: Using conditional mouse knockouts, I showed that the phosphoinositide phosphatase INPP5E shapes the lipid environment at the ciliary base, organizing actin and ensuring proteins load correctly for the trip into the outer segment.
IFT-A components (IFT43, IFTAP): I dissected how these lesser-known parts of the intraflagellar transport "fleet" govern cargo movement and long-term stability of the cilium.

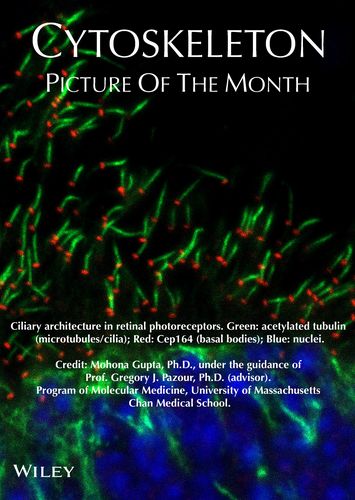
Impact:
Linking a single enzyme or transport subunit to a specific failure mode—mislocalized actin, blocked protein delivery—explains why mutations in these genes cause blindness (e.g., Joubert syndrome, MORM, retinitis pigmentosa). It also gives us defined targets to rescue before degeneration begins.
🧬 Renal Research — Kidneys & Cysts
When the Cilium's Message Doesn't Get Through
The kidney connection:
Your kidney tubule cells also carry a primary cilium—this time to monitor fluid flow and chemical cues, helping the tissue keep its architecture. In Autosomal Dominant Polycystic Kidney Disease (ADPKD), mutations in PKD1/PKD2 (polycystins) derail that ciliary signaling. Over decades, fluid-filled cysts replace healthy tissue.
The core question:
We know the polycystin complex lives in the cilium and is critical, but what exact signal is lost, and is restoring that signal enough to stop cysts?
My approach:
Precision control: I use engineered versions of the polycystin channel and a chemogenetic toolset to open the "gate" only inside the cilium, on demand.
Read the consequences: With imaging, transcriptomics, and epigenomics, I watch how cells respond—what genes flip, what pathways restore order.
Test causality: If flipping that one switch halts cyst growth in models, it's a powerful proof that targeting ciliary signaling directly can be therapeutic.
Impact:
ADPKD affects millions and has no cure. By defining the minimal signals needed to keep tubules cyst-free, we lay the groundwork for small-molecule "correctors" or gene-based strategies that restore ciliary communication rather than just managing symptoms.
⚡ Ciliary Calcium Dynamics
When a Single Ion Speaks Volumes
The calcium connection:
While the cilium hosts many messengers, calcium (Ca²⁺) is its loudest voice. Unlike the rest of the cell, the cilium is a compartment with its own rules — a nanodomain where calcium spikes are rare, confined, and deeply influential. These signals don't just flick switches. They reprogram cells.
Current focus:
In the Delling Lab, I focus on how ciliary calcium influx, particularly through the polycystin channel complex (PC1/PC2), regulates transcriptional networks that maintain tissue architecture.
My methodology:
Precision control tools: Use chemogenetic tools to control calcium entry exclusively inside the cilium, with second-by-second precision.
Multi-scale analysis: Combine this with live imaging, transcriptomics, and epigenomic profiling to map what this ionic whisper changes — from chromatin marks to gene expression.
Impact:
This work redefines calcium not just as a fast signaling ion but as a transcriptional regulator within the cilium. By showing that restoring only ciliary calcium is enough to rescue disease features in ADPKD models, we highlight it as a minimal therapeutic input — a master lever to reboot order.
📚 Publications & Impact
Research findings that advance our understanding of ciliary biology and its role in human disease.
Inpp5e is Critical for Photoreceptor Outer Segment Maintenance
Intraflagellar Transport: A Critical Player in Photoreceptor Development and the Pathogenesis of Retinal Degenerative Diseases
*Co-first authors